Patents related to COVID-19 vaccines
Infection with SARS-CoV-2 coronavirus and the resulting COVID-19 disease is affecting millions of people during the ongoing pandemic. Therefore, vaccines are needed to mitigate their effects on public health, economy, and society.
APPROVED VACCIONES AGAINST SARS-Cov-2
Research related to vaccines for this type of virus began in 2002 due to the appearance of a coronavirus in China. After the SARS-CoV-2 outbreak in 2019, efforts have been made to develop and apply vaccines that contain the spread of the disease.
Currently, the European Medicines Agency (EMA) and the Food and Drug Administration (FDA) have approved the use of two SARS-CoV-2 vaccines in the United States and Europe, respectively. The vaccines were developed by the companies Moderna and Pfizer/BioNTech and are based on a technology that has been developed since 1990. It was previously shown that the genetic material (RNA molecule) containing the information to produce the components of the virus could be used directly to generate an immune response.
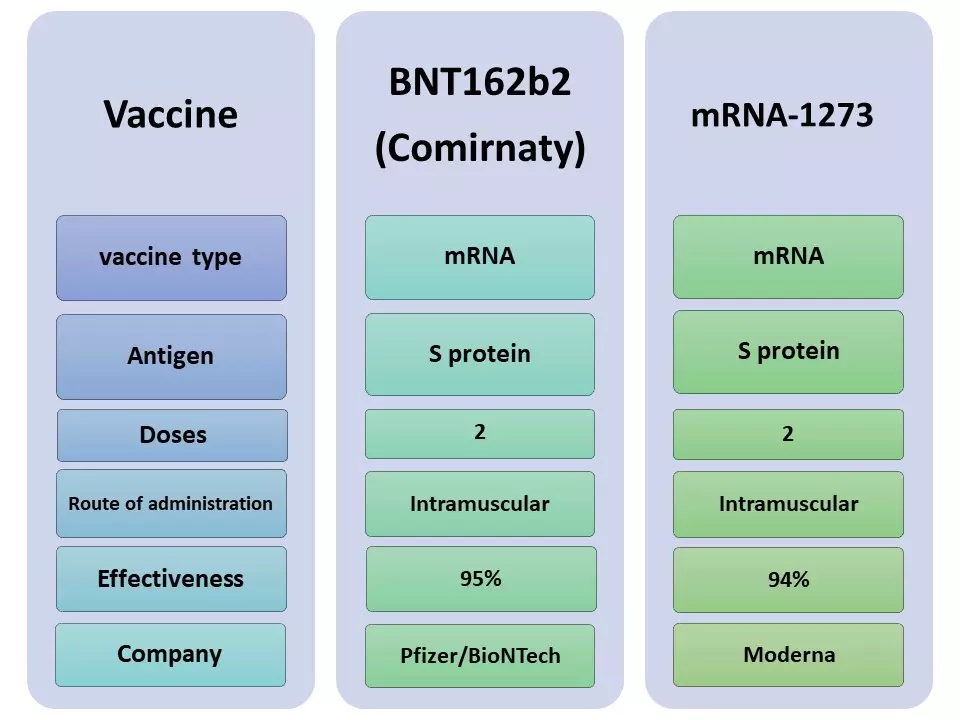
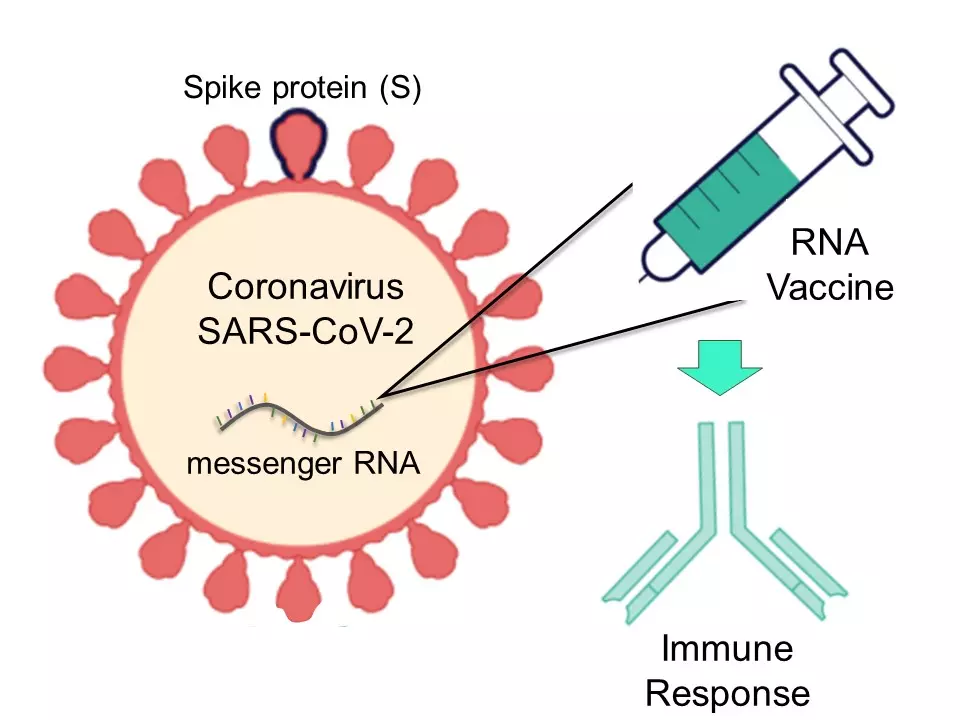
Both vaccines contain a messenger RNA (mRNA) molecule that is part of the genetic material of the virus, but it doesn't contain the virus per se. Rather, the vaccines provide a part of the virus that allows the production of a protein called Spike (protein S).
When introduced into the human body, vaccines stimulate the immune response in the subject. For example, by inducing the production of antibodies and T cells. This response helps to prepare the body in case of subsequent infection, preventing severe symptoms.
In order to carry the genetic material and release it in a stable way inside the cells, various technologies have been approached. For example, in this case that is achieved through very small capsule-like particles that enclosed and protect the molecule (mRNA).
These particles or capsules are made up of cholesterol, polyethylene glycol and other polymers. Some of these or similar components have been tested in several pharmaceutical formulations for many years.
Prior to the authorization for their massive application, the vaccines were tested in people between 16 years and up to 65 years. The effectiveness in preventing the symptoms of the disease for each formulation was demonstrated.
PATENTS RELATED TO COVID-19 VACCINES
The invention of these vaccines is based on previous research that contains elements of novelty and patentability. The pharmaceutical companies that develop it have patents on the components and technologies that are used to make the vaccine.
For example, the nanoparticle composition and its use for the delivery of messenger RNA to the cell (US Patent 9,868,692B2 and WO2016/156398). Also, methods related to the mRNA such as its synthesis, modification and use in pharmaceutical formulations (US Patent 10,064,959).
Moderna company recently obtained a patent (US Patent 10,702,600 B1 published on July 7, 2020) related to RNA vaccines targeting respiratory viruses, including SARS-CoV.
Specifically, the patent protects an invention consisting of an RNA that contains the information to generate the protein S of a coronavirus. This molecule is formulated in a lipid nanoparticle.
The patent document also describes the methods for using the vaccines, the doses, amounts, and routes of administration, among other aspects. In addition, examples of the performance of the vaccines are shown, such as their ability to induce antibody production.
Although patents are detailed technical documents, in order to learn more about each part of the invention on these vaccines, it is necessary to consult research papers that are referred throughout the description.
Given the relevance of vaccines to prevent the spread of COVID-19 disease, it is expected that new inventions will be soon patented. Follow-ups on this innovation will be in upcoming posts.
Author: Kenia Salazar Díaz (Ph.D. in Biochemistry)
